About Fuso Pharmaceutical Industries, Ltd. Company History
-
Startup phase1937 〜
-
Dialysate development1960 〜
-
Expansion of bases1975 〜
-
TSE 1st section1989 〜
-
Powder type dialysate2000 〜
-
Prime market transition2019 〜
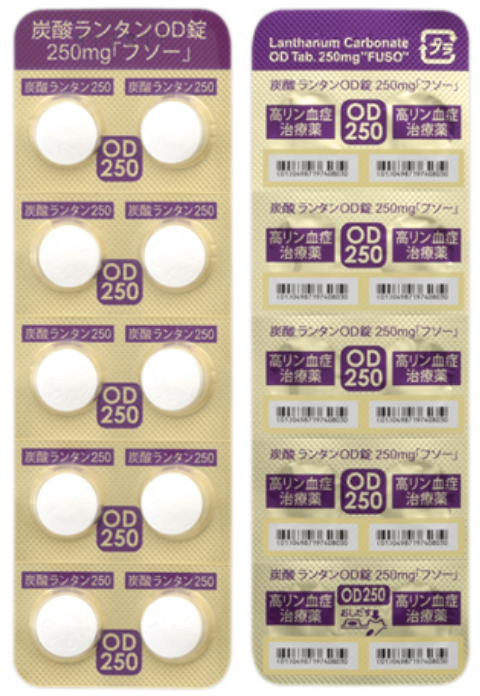

- Lanthanum Carbonate OD Tablets "FUSO"
- HiGROW OIL Heavy


Signed a co-promotion agreement with Mitsubishi Tanabe Pharma Corporation for BAFSEO Tablets, a hypoxia-inducible factor–prolyl hydroxylase inhibitor for treatment of renal anemia in dialysis.
Launched "Kindaly 5 Series" dialysate for hemodialysis.
- Kindaly AF-5 (6L,9L)
- Kindaly AF-5P
- Kindaly 5E
- Tadalafil Tablets ZA "FUSO"
- Dutasteride Capsules AV "FUSO"
- HiGROW HFF
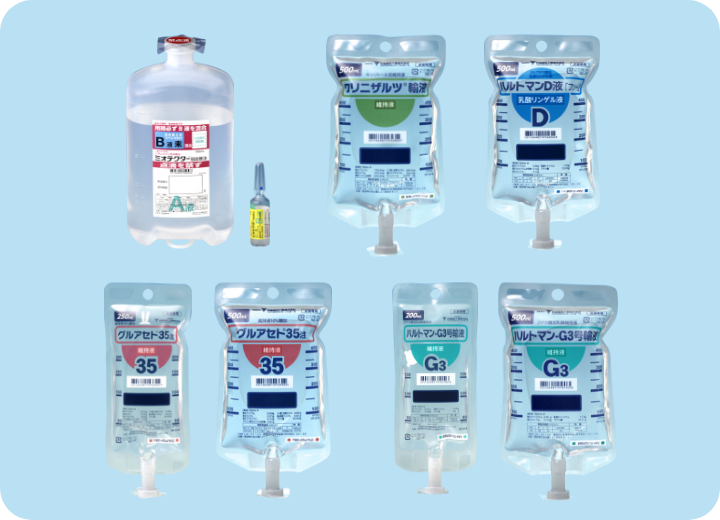

Succeeded in manufacturing and marketing approval of Miotecter, GLUACETO 35 Injection, Klinisalz, HARTMANN-G3, and Hartmann’s D SOLUTION KOBAYASHI from KYOWA CritiCare Pharma Co., Ltd. (currently neo CritiCare Pharma Co., Ltd.).
- Miotecter
- GLUACETO 35 Injection
- Klinisalz
- HARTMANN-G3
- Hartmann’s D SOLUTION KOBAYASHI


Moved ahead to be in the First Section of the Tokyo Stock Exchange, the Prime Market.
- Levocarnitine FF Syringes "FUSO"
The Maishima Distribution Center was opened at the Maishima RDC of Koyoshoji Corporation.
- DOPACOL TABLETS L250
- Argatroban HI Injection 10mg / 2mg ”FUSO”

Expanded and began production of powder dialysate for hemodialysis in the second formulation building at the Ibaraki Factory.
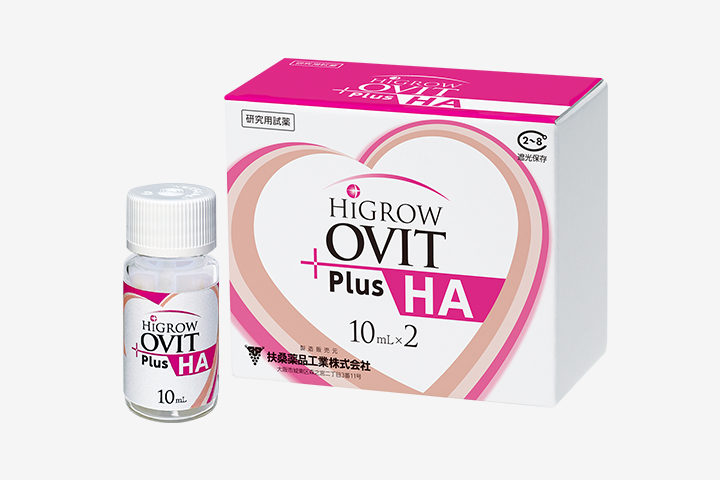
- HiGROW OVIT Plus HA
-
President's Message
-
Management Principles
-
Company History